The Medical Device Diagnostic Desk
Ecosystem
17/07/2025
A turning point for healthcare innovation: Bpifrance's Diagnostic DM window boasts a promising track record and new ambitions.
The guichet Diagnostic Dispositif Médical, operated by Bpifrance, today marks a decisive turning point in support for innovation in healthcare. This guichet, designed to support startups and SMEs in the sector in the face of complex regulations, boasts a promising balance sheet and opens up ambitious prospects for the future.
In an increasingly demanding regulatory environment for medical devices (MDs) and in vitro diagnostic medical devices (IVDMDs), the guichet Diagnostic Dispositif Médical was set up to offer strategic support. The aim is to enable innovative companies to comply with European standards, constitute their technical filesandclinically validate their products, essential steps for a successful and securemarketing.
Thus, the guichet is looking ahead to new ambitions, including the addition of two new funding streams that will be open from September 2025. These two new streams (4 and 5), will enable companies to benefit from support on cybersecurity and interoperability themes with a view to obtaining ANS certification for digital medical devices and/or referencing to the Mon espace santé service catalog, thus strengthening the support offered by the guichet.
Since 2022, Bpifrance has financed around 9M€ to support the regulatory accompaniment of 264 companies, resulting in the completion of 411 diagnostics. Of these companies, 192 operate in the digital health sector, benefiting from funding of over €5M, resulting in the completion of260 diagnostics, broken down as follows:
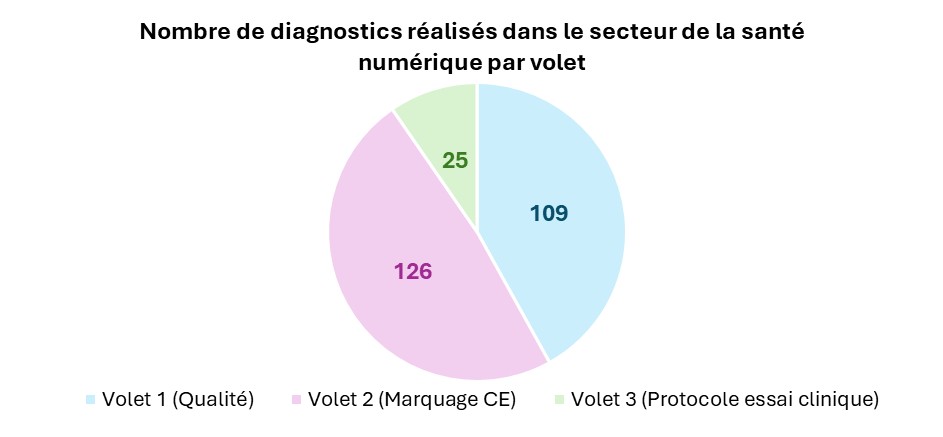
As a reminder, currently, the window covers three major components:
- Component 1: The implementation of a quality management system compliant with European standard ISO 13485, specific to medical devices.
- Part 2: The creation or upgrading of the technical documentation required to obtain CE marking for class IIa, IIb, III medical devices or class B, C, D IVDDs.
- Part 3: The design and drafting of a clinical investigation protocol to demonstrate the device's clinical and/or medico-economic benefit, a key step in promoting the product to authorities and payers.
In addition, the following two components will be added:
- Component 4: Support for cybersecurity and interoperability strategy as part of compliance with ANS referencing and referencing to Mon espace santé
- Component 5: Carrying out the cybersecurity audits required for compliance with ANS referencing standards and referencing to Mon espace santé
The funding allocated by Bpifrance, covering 50% of costs up to €110,000 excluding VAT per project, remains an essential lever for eligible companies. In this way, the Medical Device Diagnostics window has established itself as a structuring pillar of healthcare innovation in France. Its positive track record reflects an effective strategy. However, to guarantee the perennity of this scheme and encourage even wider use, it is crucial that more companies continue to make use of the guichet. We aspire for it to meet the growing needs of the ecosystem, so that it does not run out of steam, but ratherstrengthens and evolves in the face of the future challenges of healthcare innovation.
Find out more about the Medical Device Diagnostic window on the Bpifrance website.

