Technical doctrine
A shared technical doctrine to accelerate the deployment of digital healthcare services

A frame of reference
The technical doctrine for digital healthcare aims to set a frame of reference and propose a trajectory for all e-health players in France.
It is aimed at the bearers of digital healthcare services, whether they are project owners (regional e-health development support groups, healthcare establishments...) and/or prime contractors (solution publishers, integrators...) and the users of these digital services (healthcare and medico-social professionals or users of digital healthcare services in the broadest sense).
Like the "accelerating the digital shift" roadmap, this technical doctrine focuses on the exchange and sharing of healthcare data, in the healthcare and medico-social fields.
It is updated annually after public consultation.
Cross-functional projects
Electronic identification and access control
The major objectives described:
- Ensure citizens of a minimum level of guarantee in accessing their healthcare data and bring the ecosystem up to maturity.
- Ease users' access to digital healthcare services and navigation between these services.
- Discharge service providers from electronic identification, in favor of a reduced number of identity providers issuing and maintaining electronic means of identification.
- Promote referencing directories in actor identification (INS, RPPS, FINESS), a pillar of interoperability.
Useful links:
- How to integrate FranceConnect into your solution?
- Page de l'Agence du Numérique en Santé sur l'INS
- How to obtain a policyholder's INS?
- Good practices in identito-vigilance
- Le GIE SESAM-Vitale, incubateur de nouveaux usages pour l'appli carte Vitale
- Site de l'annuaire santé
- Infrastructure de gestion de la confiance " IGC-Santé "
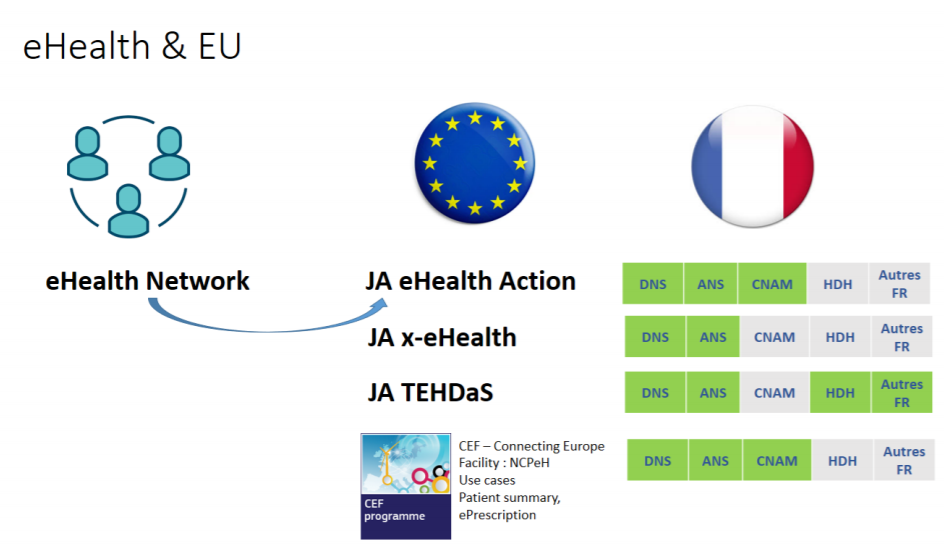
European e-health roadmap and links with the French roadmap
France participates in European coordinated actions (joint actions).
Joint eHealth Action:
- Supporting the actions of the European e-health roadmap;
- Empowering people;
- Innovative use of healthcare data;
- Improving continuity of care;
- Overcoming implementation challenges (interoperability);
- Integration into national policies and sustainability.
Joint action x-eHealth:
- Establish a common framework for patient data exchange;
- Develop the basis for a usable, interoperable, secure cross-border electronic health record exchange format.
TEDHaS (Towards a European Health Data Space) joint action:
- Create a European health data space and meet one of the European Commission's priorities for the period 2019-2025;
- Compliance with European regulations (including RGPD, eiDAS) and European directives (including the directive on cross-border care);
- Implement data exchange infrastructures between Member Countries (eHDSI) = European trusted space.
Useful links:
Espace Numérique de Santé - Package of services
Ongoing: POC CNAM - ANS Secure messaging, first results expected end 2020
Ongoing: Store " ENS: Referencing process
Since second quarter 2020: pilot experiments,
July 2021: launch of the ENS pilot version on part of the population on the DMP and MSS perimeter.
January 2022: launch of the generalization version
No later than January 2022: inclusion in the OTSS law of the automatic creation of a Digital Health Space (ENS) for everyone, unless the user or his/her legal representative objects
Useful link:


