Innovation package
Type of financing
Experimentation
Beneficiary
Medical device manufacturer
Context of use
Facility
Purpose of the system
This package allows both:
- patients to benefit from a promising technology, and
- the public authorities to ensure that the implementation of a clinical or medico-economic study will confirm the benefit for patients or the interest in terms of reducing the costs of care, and
- to the company to obtain financial support for the technology as soon as the clinical study establishing proof of its efficacy is set up.
Issues
The innovation package is a temporary financing scheme for a technology, conditional on the completion of a study that must provide clinical or medico-economic data proving its effectiveness.
- It enables a manufacturer to obtain financing for its technology in order to promote the implementation of the pivotal study that will establish its usefulness.
- It combines the early availability of a healthcare technology to patients with the collection of the clinical or medico-economic data needed to demonstrate its value with a view to its long-term financial coverage.
Is this scheme right for my project?
Dedicated to technologies with high clinical or economic stakes, entry into the innovation package scheme represents a privileged route to market.
- A technology that enters the innovation package is disseminated on the French market via the inclusion of patients in the study, with the possibility of additional patients being taken on between the end of inclusions and standard care in order to ensure continuity of care for the technology between the clinical study and standard care.
The innovation package is granted by the ministers in charge of health and social security, who examine the budgetary aspects, after receiving the opinion of the HAS, whose role is to establish the eligibility of the request with regard to: the innovative nature of the technology and the relevance of the proposed study.
Type of financing
- Temporary, derogatory coverage of the technology and the care associated with its use: for study patients and for an additional cohort in order to avoid a break in access up to standard coverage;
- The cost of the clinical study to be borne by the sponsor.
Amount of financing
The lump sum allocated is paid to the facility for each patient included in the study. It takes into account not only the technology but also the associated care costs.
Its amount is defined for each eligible technology after analysis of the projected budget drawn up by the applicant according to the model defined by the ministry. Depending on the technology, partial or total coverage is available.
For more information, visit site de la DGOS.
Calendar / periodicity
Permanent access. The company submits its application when it is ready to do so, with regard to the maturity of its technology, the first data available and when the protocol of the pivotal study that motivates its application has been established.
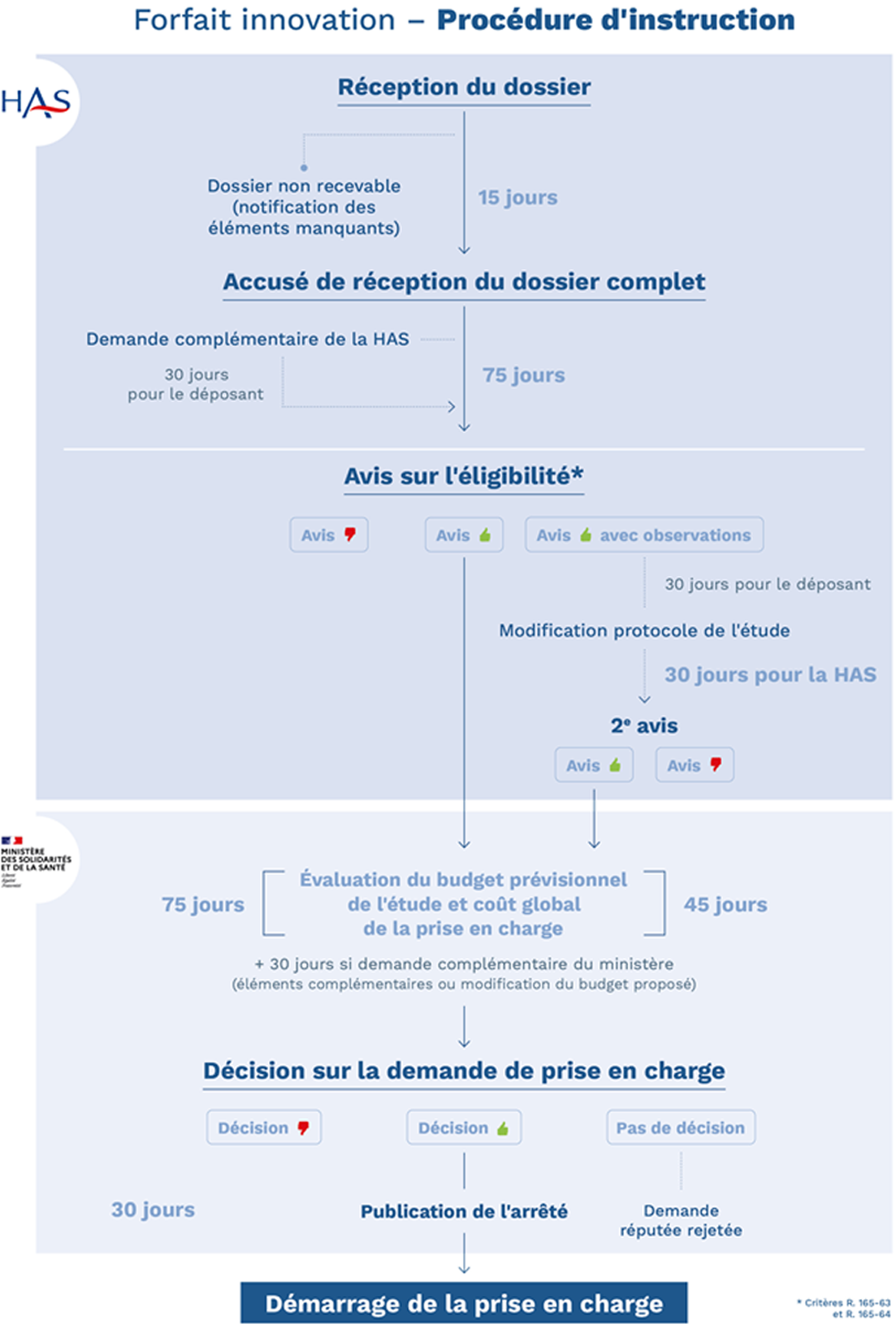
Response times are constrained by regulatory deadlines for each stage. These are now capped at 75 days for both the HAS and the Ministry, which is responsible for assessing the projected budget for the study and deciding on the overall cost of the proposed treatment.
The HAS and the Ministry are also responsible for assessing the projected budget for the study and deciding on the overall cost of the proposed treatment.
The HAS and the Ministry are also responsible for assessing the projected budget for the study and deciding on the overall cost of the proposed treatment.
Carrier type
- A distributor, a manufacturer of innovative healthcare products (medical device, in vivo diagnostic medical device);
- A national professional council (CNP) in the context of a professional act;
- In association, where applicable, with a company providing a service or with a healthcare establishment.
Project maturity
You must have already built the study protocol that will enable you to collect the data needed for standard care. This protocol is part of the dossier to be submitted.
Conditions to be met
The innovative technologies concerned are those which, at the time of filing, have data establishing that they are likely to bring a significant benefit to health or to reduce healthcare expenditure, but which are not sufficient to claim reimbursement by the community according to the rules of ordinary law.
.In short, the eligibility of a request for derogatory reimbursement is assessed by the HAS with regard to three cumulative criteria:
- the type of technology concerned with regard to the categories of healthcare technologies eligible for the innovation package, i.e. medical devices (DM), in vitro diagnostic medical devices (DM-DIV), professional acts;
- the innovative nature of the technology (4 conditions defined in article R.165-63 du code de la sécurité sociale);
- the relevance of the clinical or medico-economic study proposed by the applicant (conditions defined in article R.165-63 du code de la sécurité sociale).
How to apply
Application files must be sent simultaneously to the DGOS (Ministry of Solidarity and Health) and the HAS.
.A guide describing the dossier to be submitted and the practical procedures for submission is available to help you with the process.
Frequently asked questions
Read more
For more information, visit:
The DGOS also offers meetings, prior to the submission of applications, to discuss the general framework, how innovation support schemes work and how they interact with other financing tools.
The HAS offers industrialists the opportunity to meet (free of charge and confidential) prior to submitting their application:



