Tiers lieu: Station e-Santé
Supported by the CHU de Bordeaux
Station e-Santé helps to address the three major challenges (use, efficiency, integration)to be taken into account in the development of digital healthcare solutions in the Neurosciences, Mental Health, Cardiovascular and Surgery themes.

The Tiers lieu service offering
Station E-Santé is a Tiers-lieu for experimentation dedicated to digital health, bringing together healthcare structures, multidisciplinary scientific skills, technological and methodological skills and socio-economic structures.
The consortium is made up of several New Aquitaine playerssuch as CHU de Bordeaux (consortium leader), Centre hospitalier de la Côte Basque, Centre hospitalier de Charles Perrens, technopole UNITEC, Aquitaine Science Transfert, CATIE and Université de Bordeaux.
Station E-Santé is a regional multidisciplinary Tiers-lieu dedicated to real-life experimentation of digital health solutions for prediction, prevention/diagnosis and treatment, from idea to market, with a focus on proximity and efficiency.
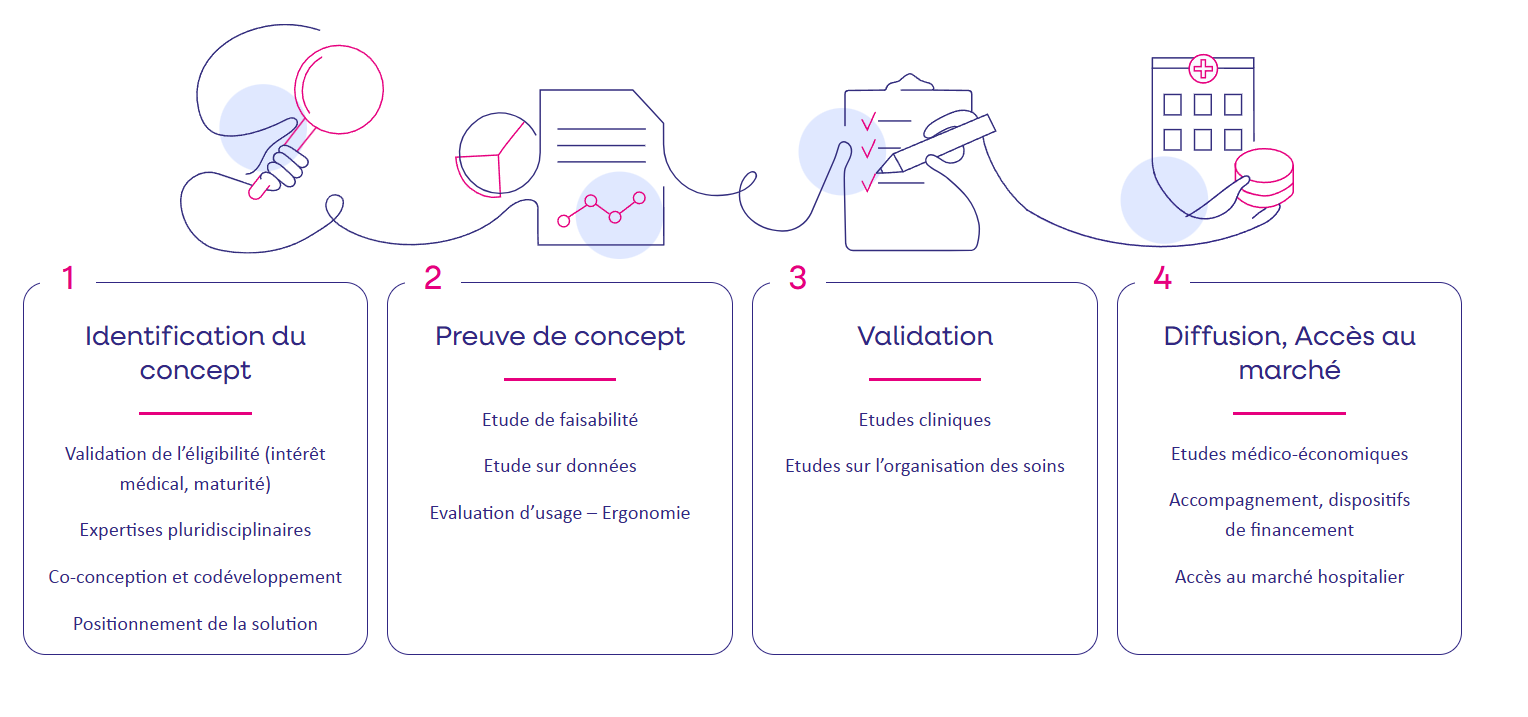
Station E-Santé deploys a range of expertise to support developers of digital healthcare solutions:
- Identification of the technological concept: multidisciplinary expertise, co-design, co-development, validation of medical interest, etc.
- Proof of concept: feasibility study, data study, usability/ergonomics assessment, etc. ;
- Validation : clinical studies, care organization studies ;
- Dissemination/market access: medico-economic studies, support for specific financing, access to the hospital market.
Tiers lieu d'expérimentation projects
Project 1: IAVASC
Solution provider : Nuréa
Solution : PRAEVAORTA software is a solution of specific algorithms enabling the automatic analysis of defined arterial zones (diameters, lengths and volumes) for the detection and follow-up of aortic aneurysms.
Need : Many patients with aortic aneurysms are not accurately detected. Moreover, current tools do not allow accurate follow-up of treated aneurysms. In order to remedy this without increasing expert interpretation time, automatic tools to obtain these complex criteria, must be provided to the practitioner.
Contribution Station e-Santé: Experimental grounds in vascular surgery and radiology, scientific and clinical expertise, clinical and imaging data collection, interpretation, scientific publication.
Project 2: KID-CCARE
Solution provider: VirtualiSurg
Solution: The KIDCCARE project aims to develop and evaluate a virtual reality simulator for managing crisis situations specific to pediatric anesthesia, designed for the initial and continuing training of physicians.
Need : The KIDCCARE project aims to co-construct and evaluate digital reality scenarios with interns and healthcare professionals, while assessing the effectiveness of this learning mode compared with conventional methods (high-fidelity simulation).
Contribution Station e-Santé: With the support of Station [e]-Santé, an evaluation of the performance and interest of simulators on complex simulated cases of pediatric anesthesia will be carried out, mobilizing anesthesia interns as well as anesthesiologists as part of their continuing education. Thanks to the involvement of CATIE, a robust methodology will also be implemented to assess the ergonomics, acceptability of simulators and interest of this learning mode for learners.
Project 3: SoQut Imaging
Solution provider : SoQut-Imaging
Solution : SoQut-Imaging offers a breakthrough method for non-invasive early-stage screening for MASH (obesity-related liver disease) and its follow-up using a liver MRI sequence.
Need: SoQut Imaging needs access to professionals in the field to validate the relevance of the solution, while enabling the co-construction of the solution leading to its experimentation.
SoQut Imaging needs access to professionals in the field to validate the relevance of the solution, while enabling the co-construction of the solution leading to experimentation.
SoQut e-Health Station's contribution: Station [e]-Health supports SoQut in demonstrating the solution's clinical added value; finalizing the development of a product that complies with regulatory and quality requirements; obtaining the various markings (CE, FDA) necessary for the marketing of a medical device.
VirtualiSurg - KID-CCARE


