Innovation santé 2030, €670m for digital health!
Innovation
09/07/2021
At the Conseil stratégique des industries de santé (CSIS) 2021, on June 29, 2021, the French President presented the plan Innovation Santé 2030. Its ambition is to make France the 1st European and sovereign nation in healthcare.
The Health 2030 innovation plan and the digital health acceleration strategy
A proactive project is being implemented with the mobilization of €7bn, including €670m for digital health as part of the France recovery plan and investments for the future. This strategy is based on 5P medicine (preventive, personalized, predictive, participative and evidence-based).
Indeed, the French healthcare system is facing important challenges such as an aging population, the development of chronic diseases, medical under-densification, questioning a medico-economic model that needs to renew itself.
France, a leader in the digital healthcare sector
France needs to position itself as a world leader in the digital healthcare sector. It needs to catch up on its lag, which can be explained by, among other things, a lack of investment in digital infrastructures, the complexity of systems created in silos, a lack of acceptability and confidence in digital technology by both the general public and professionals, a lack of visibility on market access, and a lack of training for healthcare professionals and engineers in healthcare issues.
Several major complementary public programs have already been launched in digital health, and a real momentum has thus been created:
- the roadmap for digital healthcare and the Ségur numérique program run by the French Ministry of Solidarity and Health, which aims to modernize, secure and streamline data exchanges between healthcare professionals and with patients;
- the Paris Santé Campus program, which aims to create a coherent, synergistic group of public and private operators, with the ambition of structuring a world-class digital health research and innovation sector;
- the Health Data Hub, which is helping to accelerate the exchange of data between healthcare professionals and patients.
- the Health Data Hub, which contributes to accelerating research and innovation based on healthcare data.
The digital health acceleration strategy
The actions driven by this "Digital Health" acceleration strategy aim to foster the emergence of innovative solutions, based on multidisciplinary scientific approaches and ambitious medico-economic models, to conquer the rapidly growing digital health market worldwide.
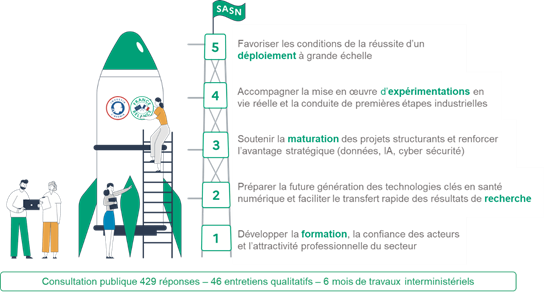
The design of the strategy is based on the results of a broad public consultation (429 responses) and targeted interviews (46 interviews), which enabled the ecosystem to express its expectations and priorities. This feedback has clarified and prioritized the content of the acceleration strategy, and is helping to design future calls for projects.
The strategy breaks down into 5 priority areas represented in chronological order of a project's life:
Examples of 5 emblematic achievements of the strategy:
1. As part of the 'Training' axis, the launch of digital health modules in initial training courses for professions in the health and medico-social sector by all training operators. As a result, 210,000 students will be trained in digital health in 24 courses and 36 universities.
2. In the 'Maturation' axis, support for the development of an imaging sector of excellence in France, by stepping up the co-financing effort for the development of new medical imaging solutions and image processing software.
.
3. As part of the 'Experimentation' axis, support the evaluation of the medicaland/or economic added value of software medical devices by funding around 50 projects by 2025.
.
4. In the 'Experimentation' axis, support for the emergence of third-party experimentation sites for digital in healthcare organizations. Thus 30 third-party sites will be opened in order to implement experiments under real conditions and the conduct of initial industrial stages.
5. In the 'Deployment' axis, launch of a reflection on the implementation of a derogatory access to digital medical devices, in order to facilitate market access for digital medical devices.

