Secondary use of health data
Innovator, start-up, researcher, you are concerned by the European health data space!
Getting started
By March 2029, the European Health Data Regulation (EEDS) will come into force for the secondary use component of health data.
In particular, it provides for a one-stop mechanism for making health data available for specific purposes such as:
- scientific research,
- innovation activities,
- and algorithm testing and evaluation.
Does this apply to me?
As an innovator, you may have several roles that give you rights and obligations:
Data holder: if you hold data for which you are a data controller. In this case:
- You must share information relating to your databases in the Répertoire national des ensembles de données (RED)
- You must make the data available to any authorized user who requests it for their project
- You can collect royalties on the provision of your data and benefit from guarantees for data protected by intellectual property and trade secret rights
Data user: If you wish to carry out processing on data and purposes listed in the regulation. In this case:
- You can find out about all the data available thanks to the RED
- You can request that data be made available via the one-stop shop.
When?
-
2027
Designation of the Organization(s) Responsible for Access to Health Data
-
2029
Most of the provisions on secondary use of health data come into force
. -
2031
Implementation of secondary use provisions for certain data: environmental determinants, "omics" data, data from clinical trials, studies and research, registry and cohort data.
Frequently asked questions
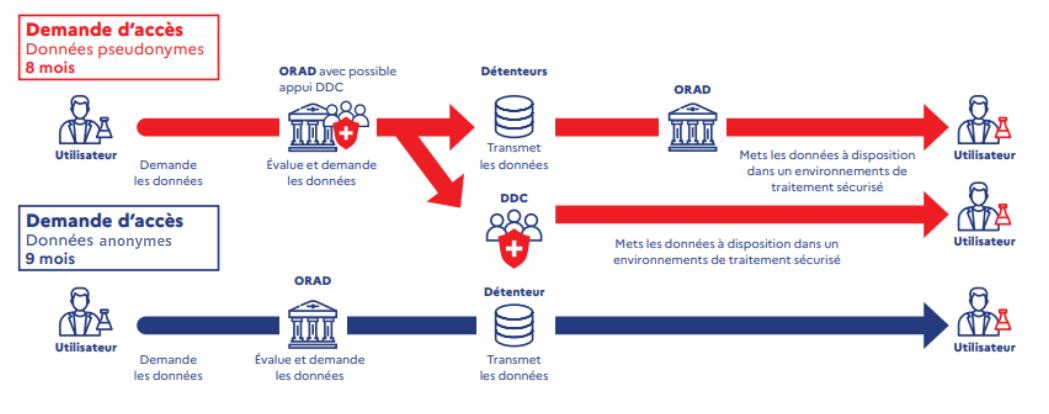
What is an Organization Responsible for Data Access (ORAD)?
The French ORAD(s) will have to be notified to the European Commission by March 2027.
The main missions of an ORAD:
Governance and licensing:
- Instructing data access requests
Preparing data pre-processing:
- Requesting data from holders; preparing data
Making data available:
- Making data available in secure environments
Standards and cooperation:
- Cooperate at national and European level on best practices, standards, etc.
HealthData@EU :
- Ensure connection and cooperation with the European HealthData@EU infrastructure
Information and transparency:
- Inform on secondary use of data; make public results, access permits, pending applications, etc.
Exercise rights :
- Fulfill obligations to individuals (rights of access, information, etc.)
Metadata catalog :
- Make available a national catalog of datasets
Fees :
- Collect data access royalties; arbitrate royalty-related disputes
Supervisions and penalties:
- Monitor data holder compliance; sanction non-compliance
What is a health data holder (HDH)?
Any DD-qualified entity will have to meet new obligations from March 2029. A DD refers to any entity that complies with the following criteria:
Condition 1:
Any natural or legal person: public authority, agency or other organization involved in the health, care or wellness sectors, including reimbursement services, developers of health products or services, producers of wellness apps, health researchers, etc.
Condition 2:
- For personal data: be responsible for processing
- For non-personal data (=anonymous data): be able to make data available via control of the technical design of a product and related services, including recording or providing such data, restricting access to such data or exchanging such data
The main missions of a DD:
Making data available:
- Make data available to ORAD on request in accordance with a processing authorization and data request
- Make non-personal data available via open and reliable databases
Remuneration:
- Estimate and collect fees for making their healthcare data available, and those covering the costs of data collection and preparation
Metadata and discovery:
- Communicate a description of its datasets to ORAD and update the data listed on the catalog at least once a year within 3 months
- Provide documents to ORAD for the quality and usefulness label
What is a trusted data holder (TDS)?
France must set up a designation procedure for a DD to become a DDC. The DDC will have to meet at least three conditions:
- Give access to DDs by means of a compliant secure processing environment
- Have the necessary expertise to assess requests for access to health data and requests for health data
- Provide the necessary guarantees regarding compliance with the regulation.
The main missions of a DDC:
Governance & licensing :
- Instructing and making recommendations on access requests
Making data available:
- Make available in a secure processing environment (personal data)
- Make available the results of a query (anonymous data)
HealthData@EU:
- Facilitate cross-border access
Fees:
- Collect fees
Experts
Secondary use of healthcare data: A revolution for innovation and research in France
The secondary use of health data today represents a major challenge for medical innovation and scientific research. With the forthcoming entry into force of the European Health Data Space (EHDS) regulation by March 2029, France is positioning itself as a key player in this digital transformation.
The European regulatory framework: EHDS and national strategy
The European Health Data Space (EHDS) regulation therefore provides the legal foundation for this development. In France, this regulation is accompanied by an interministerial strategy launched in 2025, structured around four priority areas:
- Fostering transparency and citizen confidence
- Constituting reusable bases of interest
- Gathering the necessary conditions for the sharing and reuse of health data
- Enabling and simplifying the use of health data
The latter aims to optimize the reuse of health data while preparing for the application of the EEDS regulation. This coordinated approach involves many players: the DNS, the CNIL, the Health Data Hub, and the CNAM.
Obligations of health data holders within the European framework of data reuse
Health data holders, will have to share information relating to their bases in the Répertoire national des ensembles de données (RED). This is in line with the European regulation on data reuse. They will have to allow secondary use of health data to any user who requests it for their research, care or digital development projects. These holders will become key players in the national and European strategy for making the most of data health, in line with CNIL recommendations and the objectives of the European Health Data Space.
Access, processing and reuse of health data: a digital strategy for research, innovation and personalized care
The secondary use of health data represents a major opportunity for innovation players, researchers and healthcare professionals. Users, wishing to carry out a processing of personal health data, can consult the data available via the RED and make a request for provision via the one-stop shop.
These data, essential for processing and analysis, can be reused as part of research projects, digital health innovation or to improve patient care. The European Health Data Space facilitates cross-border collaboration, particularly in research into rare diseases, and reinforces France's global and European strategy for quality reporting and analysis.
The exploitation of vast volumes of data health enables the development of artificial intelligence algorithms, promoting more accurate diagnoses and personalized patient treatment. This availability of data thus opens up new prospects for innovation, while guaranteeing a secure and ethical framework.
Valuing health data: a balance between innovation, European framework and patients' rights
Valuing health data for secondary use relies on an essential balance between digital innovation and the protection of patients' rights. The European regulation provides for a fee system for data holders, recognizing the value of making data available within a secure framework. This data can be reused to fuel research projects, health innovation initiatives, or evaluative studies, contributing to the improvement of care and the construction of a national and European digital health strategy.








